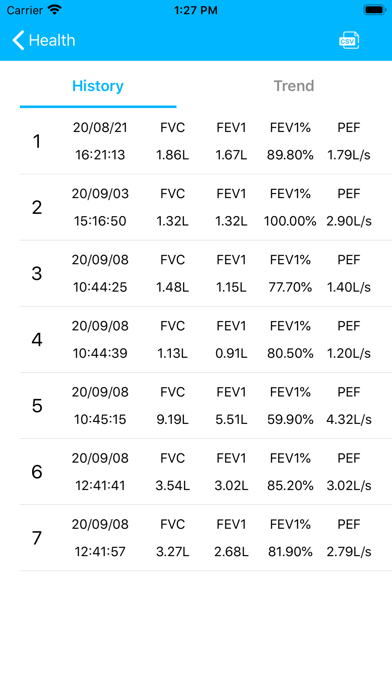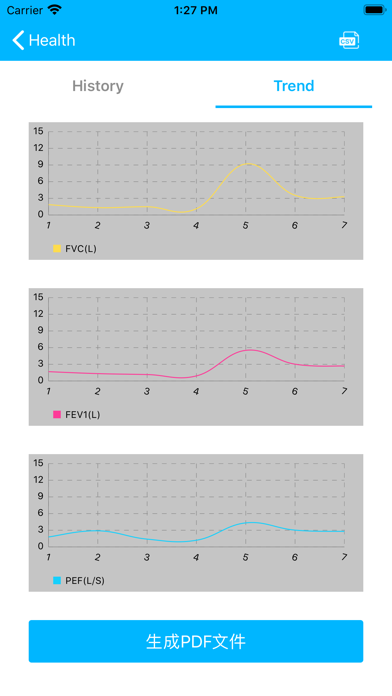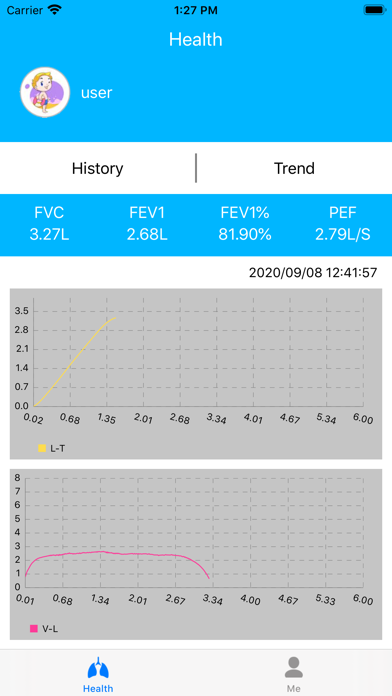
Lung Doctor is an application for users with abnormal lung function such as asthma and chronic obstructive pulmonary disease. Users can cooperate with Kangtai Medicals spirometer to test their lung function status, and to understand the test data and lung function parameter trends in detail, to facilitate patient self-monitoring and management and doctors to check the condition.
Our device has passed the CFDA certification and the European Union CE certification.
CFDA registration certificate number: 冀械注准 20172200014
CE No.G1 050972 0050 Rev.04 Report No.:BJ20090203
【Disclaimer】
Users should consult a doctors advice before making any medical decisions using the App.
Health consultation answers are only used as health consultation suggestions for users reference, but not as the basis for diagnosis, diagnosis and treatment, nor as any legally valid written certification. If the user uses this as the basis for diagnosis and treatment, any negative impact or even loss caused by it will be the responsibility of the questioner himself, and the platform will not be responsible.
【Jurisdiction Statement】
We control and operate this App in countries and regions such as China and the European Union. The hardware devices connected to the app are only licensed in China and EU countries.
We make no representations about the applicability of any material on this App for any other location. If you choose to access the App from other locations, you are responsible for compliance with applicable local laws.